Colby Stong
Omalizumab resulted in significantly lowered OCS use in 65% of patients with ABPA and eliminated OCS use in 53% of patients.
Omalizumab was associated with a significant decrease in the annual exacerbation rate and use of oral corticosteroids (OCS) in patients with allergic bronchopulmonary aspergillosis (ABPA), according to meta-analysis findings in the Journal of Allergy and Clinical Immunology: In Practice.
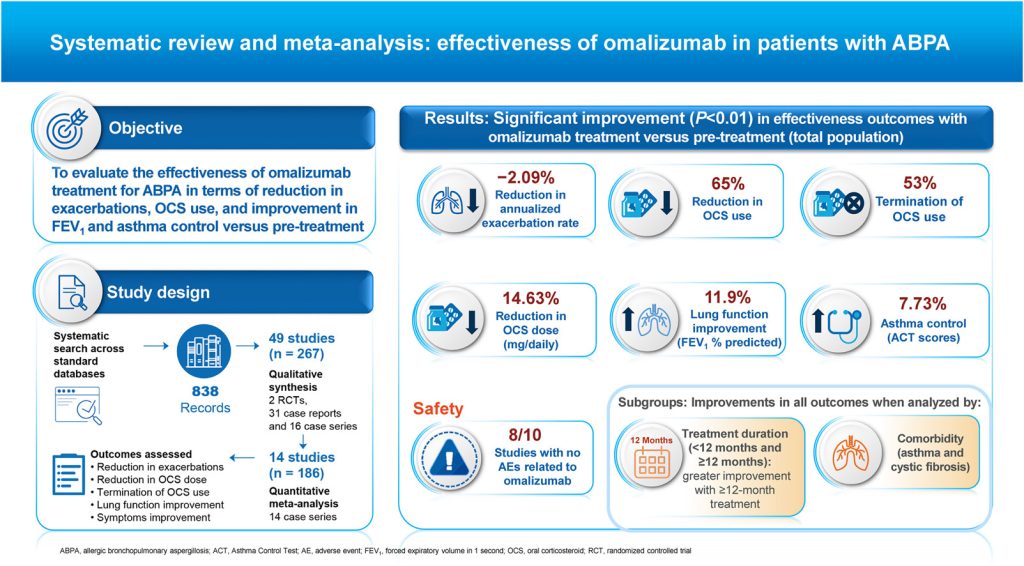
The systematic review and meta-analysis assessed the potential clinical benefits of omalizumab in patients with ABPA and tested the hypothesis that treatment with omalizumab leads to clinically meaningful improvement in symptoms, reduces the use of OCS, and improves forced expiratory volume in 1 second (FEV1).
Investigators searched the PubMed, Embase, Biosis (Ovid), Web of Science, and Cochrane Central Register of Controlled Trials databases from inception to May 13, 2021, to identify articles that evaluated omalizumab as monotherapy or in adjunct to systemic steroids or antifungal treatment among patients with ABPA.
The primary outcomes were a decrease in annualized exacerbation rate, reduction in or termination of OCS use and reduction in OCS dose, lung function improvement (FEV1 percent predicted), and asthma control per the asthma control test (ACT).
A total of 49 studies with 267 patients were included in the qualitative synthesis (2 randomized controlled trials, 31 case reports, and 16 case series), and 14 case series (n=186; 12 retrospective and 2 prospective studies) were included in the quantitative (meta-analysis) synthesis. Most of the studies were conducted in Europe and Asia, and treatment duration with omalizumab ranged from 4 to 36 months.
In the quantitative meta-analysis, exacerbation data were obtained from 3 studies (n=37). Omalizumab significantly reduced the annualized exacerbation rates in patients with ABPA at 8.3 to 12 months vs baseline, with a mean difference of -2.09 (95% CI, -3.07 to -1.11; P <.01 for both subgroups).
Among 8 studies that assessed the OCS-sparing effect (n=84), 4 included ABPA patients with asthma, and 4 included patients with cystic fibrosis. The binary outcomes analysis showed that omalizumab significantly lowered OCS use vs pretreatment in 65% of patients (risk difference [RD], 0.65; 95% CI, 0.46-0.84; P <.01) for treatment of 1 to 20 months. Also, 53% of participants stopped OCS use after treatment with omalizumab for 6 to 54 months (RD, 0.53; 95% CI, 0.26-0.80; P <.01).
The decrease in daily OCS dose with omalizumab treatment was assessed in 5 studies (n=64). A significant decrease in the mean daily OCS dose of 14.62 mg (95% CI, -19.86 to -9.39; P <.01) was found in patients with ABPA who received omalizumab for 4 to 54 months.
A total of 9 studies reported findings on FEV1 percent predicted (n=120). A significant improvement in mean FEV1 percent predicted was found in patients with ABPA treated with omalizumab for 6 to 54 months (mean difference, 11.9; 8.2-15.6; P <.01).As many patients with ABPA might be candidates for long-term OCS treatment, the ability of omalizumab to reduce or cease OCS use is clinically meaningful, suggesting a valuable role of the anti-immunoglobulin E therapy in the management of treatment-refractory ABPA.
A statistically significant improvement of 7.73 in ACT score (95% CI, 4.88-10.58; P <.01) during omalizumab treatment for 3.7 to 54 months was observed in 3 studies (n=24).
Among 10 studies (n=151) that included adverse events (AEs), 8 (n=94) did not report any significant AEs related to omalizumab and 5 mild AEs were observed in the other 2 studies.
Study limitations include the use of case series data leading to selection bias and substantial heterogeneity, as well as the absence of a placebo or control group. Also, the sample size was relatively small, which may limit the statistical power of the analysis. Furthermore, baseline clinical data regarding stage and duration of ABPA and extent of bronchiectasis were not available for most patients.
“As many patients with ABPA might be candidates for long-term OCS treatment, the ability of omalizumab to reduce or cease OCS use is clinically meaningful, suggesting a valuable role of the anti-immunoglobulin E therapy in the management of treatment-refractory ABPA,” the investigators concluded.
Disclosure: Medical writing support for the manuscript was funded by Novartis Pharma AG, Basel, Switzerland. Molecular Connections Pvt Ltd provided support in the data analyses. Some of the study authors declared affiliations with biotech, pharmaceutical, and/or device companies. Please see the original reference for a full list of authors’ disclosures.
References:
Jin M, Douglass JA, Elborn JS, et al. Omalizumab in allergic bronchopulmonary aspergillosis (ABPA): a systematic review and meta-analysis. J Allergy Clin Immunol Pract. Published online December 26, 2022. doi:10.1016/j.jaip.2022.12.012
