
Pesantes, E., Hernando, R., Lores, C. et al. BMC Pulm Med 24, 421 (2024). https://doi.org/10.1186/s12890-024-03227-y
Abstract
Background
Fractional exhaled nitric oxide (FeNO) is used for the diagnosis and monitoring of asthma, although its utility to guide treatment and its correlation with other tools is still under discussion. We study the possibility to withdraw inhaled corticosteroid treatment in atopic patients with mild asthma based on the FeNO level, as well as to study its correlation with other clinical control tools.
Methods
Prospective and randomized study including atopic patients aged 18 to 65 with mild asthma, stable, on low-dose inhaled corticosteroid (ICS) treatment, who had their treatment withdrawn based on a FeNO level of 40 ppb. Patients were randomized into two groups: control group (treatment with ICS was withdrawn regardless of FeNO level) and experimental group (according to the FeNO levels, patients were assigned to one of two groups: FeNO > 40 ppb on treatment with budesonide 200 mcg every 12 h and SABA on demand; FeNO ≤ 40 ppb only with SABA on demand). Follow-up was conducted for one year, during which medical assessment was performed with FeNO measurements, asthma control test (ACT), lung function tests (FEV1, FEV1/FVC, PEF, and RV/TLC), and recording of the number of exacerbations.
Results
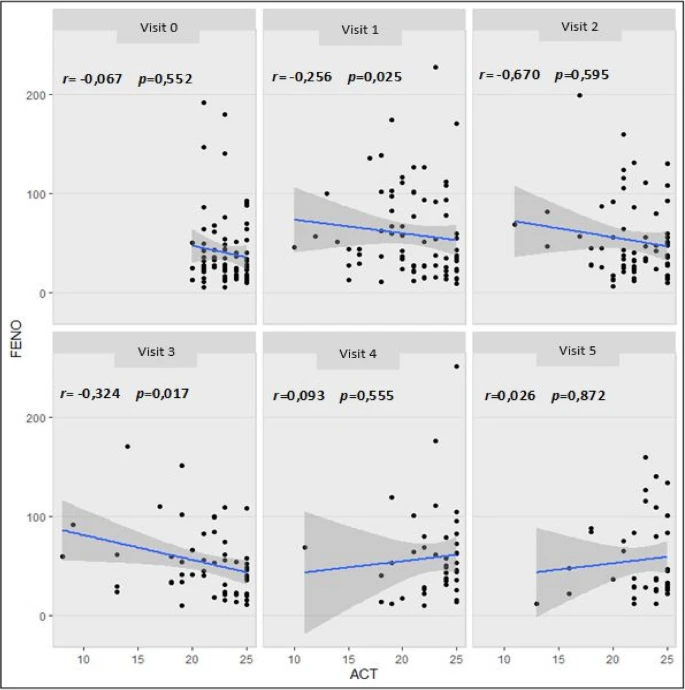
Ninety-two patients were included, with a mean age of 39.92 years (SD 13.99); 46 patients were assigned to the control group, and 46 patients to the experimental group. The number of exacerbations was similar between the groups (p = 0.301), while the time to the first exacerbation was significantly shorter in the control group (30.86 vs. 99.00 days), p < 0.001, 95% CI (43.332—92.954). Lung function tests (FEV1, FEV1/FVC, PEF, and RV/TLC) showed no differences between the groups (p > 0.05). Both FeNO and ACT showed significant changes in the groups in which ICS was withdrawn (p < 0.05 for both parameters). A significant negative correlation was observed between FeNO and ACT (r = -0.139, p = 0.008).
Conclusions
In atopic patients with mild asthma, withdrawal of ICS based on an FeNO of 40 ppb led to worsened symptoms but without changes in lung function tests or an increase in exacerbations. There was a negative correlation between FeNO values and symptomatic control measured by the ACT.
