Jesenak, M., Hrubisko, M., Chudek, J. et al. Sci Rep 15, 7146 (2025). https://doi.org/10.1038/s41598-025-91830-2
Abstract
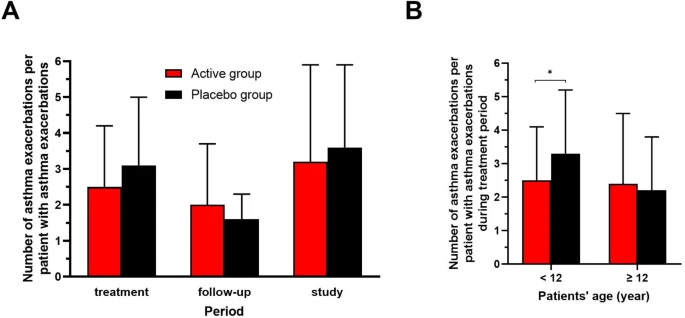
The aim of this study was to evaluate the effects of pleuran (β-glucan isolated from Pleurotus ostreatus) on asthma control and respiratory morbidity in children on conventional GINA-based asthma treatment who had partially controlled perennial asthma. A double-blind, placebo-controlled multicentre clinical trial with a 2-arm, parallel design was conducted across three countries; 230 children aged 7 to 17 years were randomised (1:1) into an active group (receiving a pleuran/vitamin C combination) or a placebo group (receiving vitamin C only). This study consisted of 24 weeks of treatment (2 capsules a day) and then 24 weeks of follow-up. The primary endpoints included the effects of active treatment versus placebo on asthma control and respiratory tract infections (RTIs). Secondary endpoints included changes in the following measures: number of asthma exacerbations, with or without respiratory infection; quality of life of both asthmatic children and their caregivers; spirometric indices; fractional exhaled nitric oxide (FeNO) levels; safety after 24 weeks of treatment and also after the full 48-week study period. Overall, 206 children completed this study; 113 of these children were in the active group and received a pleuran/vitamin C combination for 24 weeks. After the 24-week treatment period, children below 12 years of age who were in the active group achieved significant improvements in asthma control compared to those in the placebo group (21.8 ± 3.5 vs. 20.3 ± 4.0; P = 0.02); while children at least 12 years old who were in the active group reported lower numbers of RTIs (0.7 ± 1.0 vs. 1.9 ± 1.7; P = 0.002) compared to children of this age in the placebo group. In addition, children below 12 years of age in the active group showed a significant decrease in asthma exacerbations compared to those in the placebo group (2.5 ± 1.6 vs. 3.3 ± 1.9; P = 0.05). At the end of the 48-week trial, a statistically significant improvement in asthma control was observed in 84.7% of children who received pleuran/vitamin C treatment compared to 67.0% of children who received vitamin C only (P = 0.01). The pleuran/vitamin C combined treatment was safe and well-tolerated, and no related serious adverse events were reported. This study highlights the favourable safety profile of pleuran/vitamin C supplementation and demonstrates positive effects of this treatment on asthma control and RTI incidence in children with allergic perennial asthma that was partially controlled by conventional therapy.
