
Rahimi, B., Khoshnam Rad, N., Amini, S. et al. BMC Pulm Med 25, 167 (2025) https://doi.org/10.1186/s12890-025-03629-6
Abstract
Background
While asthma guidelines advocate for reducing inhaled corticosteroid (ICS) doses in well-controlled patients, limited evidence exists to directly support this approach. This study aimed to compare the effectiveness of ICS dose reduction versus montelukast discontinuation as step-down strategies in adults with well-controlled asthma.
Methods
This single-center, pilot randomized controlled trial enrolled 73 adults with well-controlled asthma. Participants were randomized to either Group A: ICS Dose Reduction (n = 37) or Group B: Montelukast Discontinuation (n = 36). Both groups received standard care and their designated intervention for three months. The primary outcome was asthma control measured by the ACT score. Secondary outcomes included lung function, asthma exacerbation frequency, treatment failure rates, and cough symptoms. Medication adherence was assessed using dose counters and pill counts.
Results
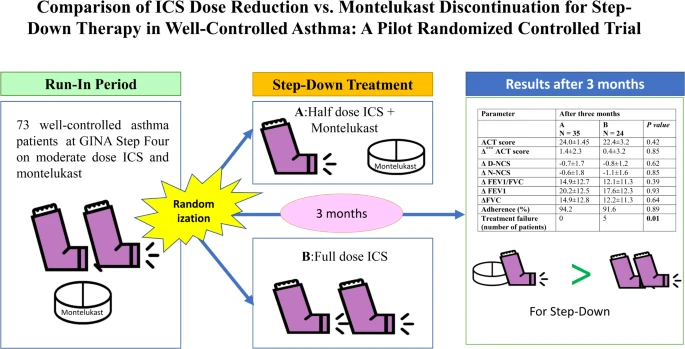
There was no significant difference in overall asthma control between the groups, as measured by the ACT score (p = 0.42). However, patients in Group A (reduced ICS) experienced significantly fewer treatment failures compared to Group B (discontinued montelukast) at three months (p = 0.01). No serious adverse events were reported.
Conclusion
Although the ACT scores did not significantly differ between the groups, we did observe a trend towards fewer treatment failures in the ICS reduction group. This suggests that reducing ICS doses may help to maintain asthma control and reduce the risk of exacerbations. However, further research is warranted to confirm these findings in larger, long-term studies.
