
Gao, Y., Liang, B., Su, X. et al. BMC Pulm Med 25, 228 (2025). https://doi.org/10.1186/s12890-025-03690-1
Abstract
Background
Access to spirometry remains limited due to the expense and inconvenience of stationary laboratory spirometers, which may compromise the diagnosis and management of chronic respiratory diseases (CRDs), such as chronic obstructive pulmonary disease (COPD) and asthma. Portable spirometers offer potential advantages over laboratory spirometers in terms of affordability, user-friendliness, and portability. The objective of this study is to evaluate the reliability and usability of a portable spirometer (Medcaptain VC-30 Pro) compared to a conventional laboratory spirometer (Jaeger MasterScreen PFT).
Methods
In this multi-center, randomized, open-label crossover study, 132 subjects from two hospitals were recruited to perform pulmonary function tests using both the portable spirometer and the laboratory spirometer. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, peak expiratory flow (PEF), forced expiratory flow between 25 and 75% of FVC (FEF25-75%), vital capacity (VC), maximal voluntary ventilation (MVV), and forced expiratory volume in six seconds (FEV6) were compared for correlation and agreement between two spirometers. The concordance of their spirometric abnormality diagnoses and severity classifications was assessed. An additional 30 healthy volunteers were recruited to perform a pulmonary function test by themselves after a session guided by specialists to evaluate the usability of the portable spirometer.
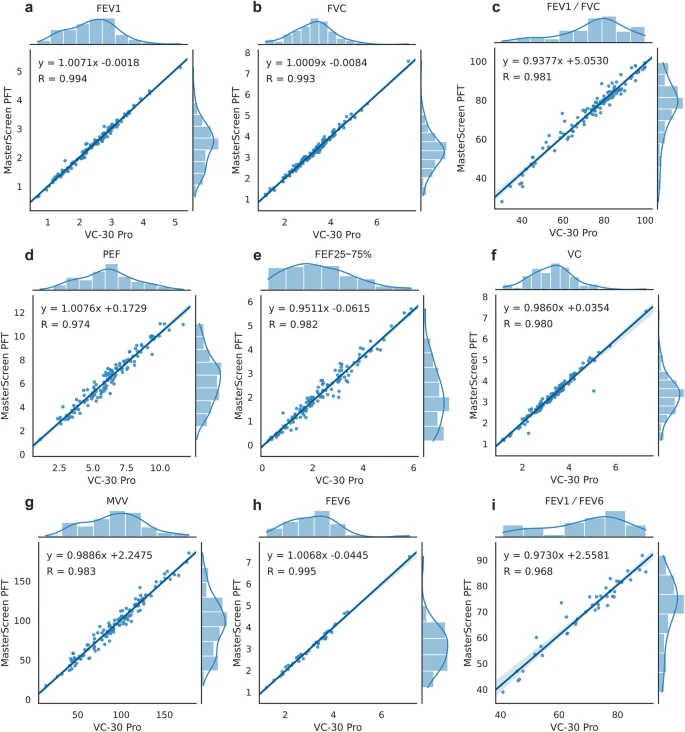
Results
A total of 126 recruited participants achieved acceptable pulmonary function test results. The intraclass correlation coefficients (ICCs) for primary outcomes FEV1 and FVC were 0.994 and 0.993, respectively (both p < 0.001). ICCs for other outcomes ranged from 0.968 to 0.995 (all p < 0.001). The Bland–Altman analysis showed that FEV1 and FVC met preset acceptable criteria, with 96.0% of values falling within the 95% limits of agreement (LoA). Cohen’s kappa statistics for the diagnosis of spirometric abnormality and classification of severity were 0.872 and 0.878, respectively. In the usability test, 28 out of 30 volunteers obtained a Grade A result.
Conclusions
The portable spirometer exhibited a strong correlation and agreement with a high-quality laboratory spirometer, as well as concordance in spirometric abnormality diagnosis and severity classification. Non-specialist can obtain acceptable results using this portable spirometer.
