Article – Open Access
Zbigniew Zasłona, Ewelina Flis, Mieszko M. Wilk, Richard G. Carroll, Eva M. Palsson-McDermott, Mark M. Hughes, Ciana Diskin, Kathy Banahan, Dylan G. Ryan, Alexander Hooftman, Alicja Misiak, Jay Kearney, Gunter Lochnit, Wilhelm Bertrams, Timm Greulich, Bernd Schmeck, Oliver J. McElvaney, Kingston H. G. Mills, Ed C. Lavelle, Małgorzata Wygrecka, Emma M. Creagh & Luke A. J. O’Neill
Nature Communications volume 11, Article number: 1055 (2020)
Abstract
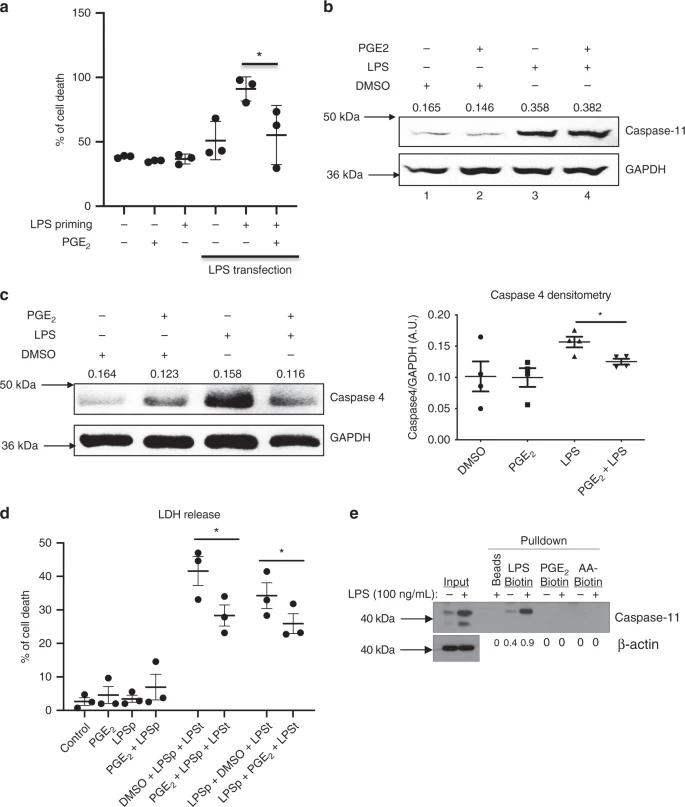
Activated caspase-1 and caspase-11 induce inflammatory cell death in a process termed pyroptosis. Here we show that Prostaglandin E2 (PGE2) inhibits caspase-11-dependent pyroptosis in murine and human macrophages. PGE2 suppreses caspase-11 expression in murine and human macrophages and in the airways of mice with allergic inflammation. Remarkably, caspase-11-deficient mice are strongly resistant to developing experimental allergic airway inflammation, where PGE2 is known to be protective. Expression of caspase-11 is elevated in the lung of wild type mice with allergic airway inflammation. Blocking PGE2 production with indomethacin enhances, whereas the prostaglandin E1 analog misoprostol inhibits lung caspase-11 expression. Finally, alveolar macrophages from asthma patients exhibit increased expression of caspase-4, a human homologue of caspase-11. Our findings identify PGE2 as a negative regulator of caspase-11-driven pyroptosis and implicate caspase-4/11 as a critical contributor to allergic airway inflammation, with implications for pathophysiology of asthma.
