Rudin RS, Plombon S, Sulca Flores J, et al. JAMA Netw Open. 2025;8(4):e256219. doi:10.1001/jamanetworkopen.2025.6219
Key Points
Question Does a scalable asthma symptom monitoring intervention improve asthma-related quality of life?
Findings In this randomized clinical trial involving 413 patients across 7 primary care clinics, a mobile health–based intervention using weekly questionnaires led to a statistically significant but not clinically meaningful increase in patient-reported asthma-related quality of life. There was no change in nonroutine asthma-related health care utilization, although patients with low levels of self-assessed activation (defined by knowledge, skills, and confidence in managing their health) may derive perceptible benefit.
Meaning These findings did not reach the threshold for a minimally important change in asthma-related quality of life with a scalable asthma symptom monitoring intervention; however, subgroups of patients, particularly those with low levels of self-assessed activation, may experience benefit.
Abstract

Importance Asthma affects an estimated 7.7% of the US population and 262 million people worldwide. Symptom monitoring has demonstrated benefits but has not achieved widespread use.
Objective To assess the effect of a scalable asthma symptom monitoring intervention on asthma outcomes.
Design, Setting, and Participants This randomized clinical trial was conducted between July 2020 and March 2023 at 7 primary care clinics affiliated with an academic medical center (Brigham and Women’s Hospital in Boston, Massachusetts). Candidate patients with a diagnosis of asthma over a 20-month recruitment period (July 2020 to March 2022) were identified and categorized into tiers of varying disease activity based on electronic health record data. Eligible patients were adults (aged ≥18 years) and had a primary care practitioner in 1 of the 7 participating clinics.
Intervention Intervention group patients were asked to use a mobile health app to complete weekly symptom questionnaires; track notes, peak flows, and triggers; and view educational information. Patients who reported worsening or severe symptoms were offered clinical callback requests. App data were available in the electronic health record. Usual care group patients received general asthma guidance.
Main Outcomes and Measures The primary outcome was the mean change in Mini Asthma Quality of Life Questionnaire (MiniAQLQ) score for the intended 12-month study period. A change of 0.5 on a scale of 1 to 7 was considered a minimally important change. The secondary outcome was the mean number of asthma-related health care utilization events (urgent care visits, emergency department visits, or hospitalizations). Mean differences for all outcomes between groups were compared using robust linear regression models (generalized estimating equations) with treatment group as the only covariate.
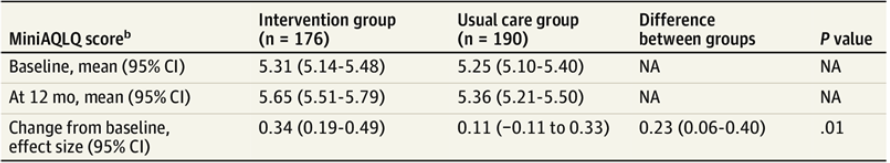
Results Baseline questionnaires were completed by 413 patients (mean [SD] age, 52.2 [15.4] years; 321 women [77.7%]). Of these, 366 patients completed final questionnaires and were included in the primary analysis. MiniAQLQ scores increased 0.34 (95% CI, 0.19-0.49) in the intervention group and 0.11 (95% CI, −0.11 to 0.33) in the usual care group from baseline to final questionnaire completion (adjusted difference-in-difference, 0.23 [95% CI, 0.06-0.40]; P = .01); although the difference was statistically significant, it did not reach the threshold for a minimally important change. Intervention subgroups showed positive differences in MiniAQLQ scores relative to the usual care group, with noteworthy increases among individuals aged 18 to 44 years (adjusted difference-in-difference, 0.40 [95% CI, 0.13-0.66]), those with low baseline patient activation (adjusted difference-in-difference, 0.77 [95% CI, 0.30-1.24]), those with a low baseline MiniAQLQ score (adjusted difference-in-difference, 0.33 [95% CI, 0.07-0.59]), and those with uncontrolled asthma at baseline (adjusted difference-in-difference, 0.30 [95% CI, 0.05-0.54]). The intervention group had a mean of 0.59 (95% CI, 0.42-0.77) nonroutine asthma-related utilization events compared with 0.76 (95% CI, 0.55-0.96) in the usual care group (adjusted effect size, −0.16 [95% CI, −0.42 to 0.17]; P = .23).
Conclusions and Relevance In this randomized clinical trial of a scalable symptom monitoring intervention, the increase in asthma-related quality of life did not reach the threshold for a minimally important change. Exploratory analyses suggest possible benefits for patients with low levels of activation.
