Research | OPEN |
Respiratory Research
David M. G. Halpin, Eli O. Meltzer, Wendelgard Pisternick-Ruf, Petra Moroni-Zentgraf, Michael Engel, Liliana Zaremba-Pechmann, Thomas Casale & J. Mark FitzGerald
Abstract
Background
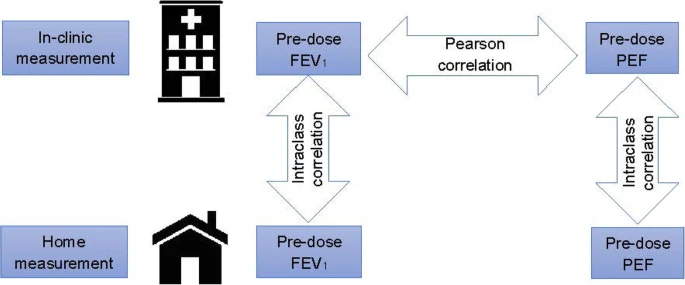
The primary lung function endpoint in clinical trials in adolescent and adult patients with asthma is usually forced expiratory volume in one second (FEV1). The objective of our analysis was to assess whether peak expiratory flow (PEF) is a suitable alternative primary lung function endpoint.
Methods
For this assessment, we calculated post hoc the correlation between pre-dose FEV1and pre-dose PEF measured under supervision in the clinic and, for both lung function parameters, the correlations between supervised clinic and unsupervised home measurements, using the results from the 8 Phase III parallel-group trials of the global clinical development programme with tiotropium Respimat® in patients with asthma aged 12 to 75 years.
Results
Across all 8 trials included in this analysis, changes in lung function from baseline correlated well between pre-dose FEV1 and pre-dose PEF when both were measured under supervision in the clinic. Correlation between supervised in-clinic and unsupervised home measurements was stronger for pre-dose PEF than for pre-dose FEV1.
Conclusions
Pre-dose PEF measured at home could be an alternative primary lung function endpoint for trials in adolescent and adult patients with asthma. Using home-measured PEF could facilitate trial conduct and improve the convenience for patients by relocating scheduled assessments from the clinic to the patient’s home.
Trial registration
Adolescents aged 12 to 17 years: RubaTinA-asthma® (NCT01257230), PensieTinA-asthma® (NCT01277523).
Adults aged 18 to 75 years: GraziaTinA-asthma® (NCT01316380), MezzoTinA-asthma® (NCT01172808/NCT01172821), CadenTinA-asthma® (NCT01340209), PrimoTinA-asthma® (NCT00772538/NCT00776984).
All from Clinicaltrials.gov (https://clinicaltrials.gov/).
